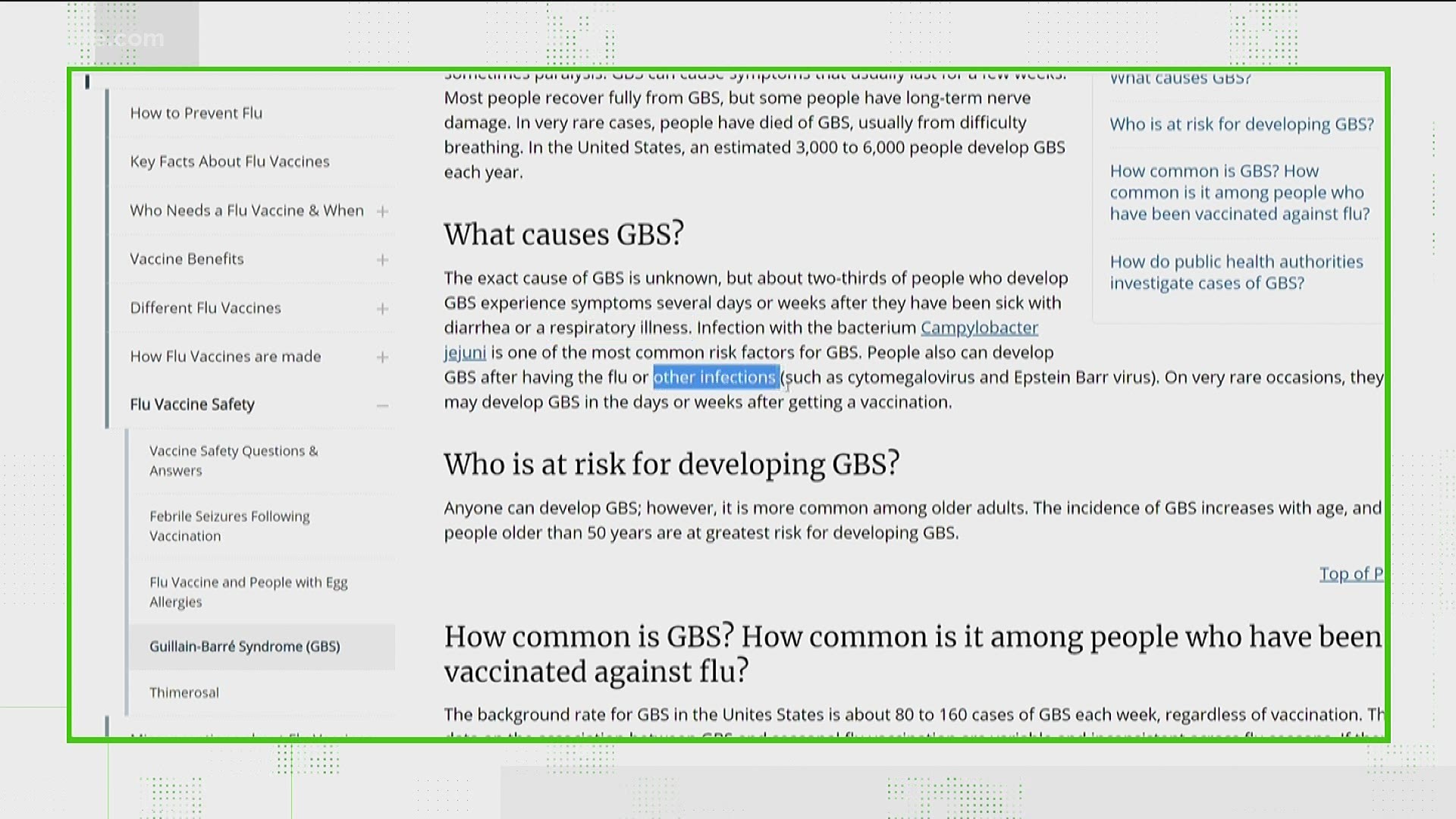
Other vaccines have been associated with GuillainBarré syndrome Although the Johnson & Johnson shot is the only COVID19 vaccine that has been linked to GuillainBarré syndrome, it's not the Regulators say the benefits of the vaccine still outweigh any risks GuillainBarré syndrome (GBS) is a rare neurological disorder in which the immune system attacks the nerves It can cause muscle weakness and sometimes paralysis People usually recover from it, but it can lead to hospitalization and, sometimes, permanent damage to nerve cells "Reports of adverse events following use of the Janssen COVID19 Vaccine under emergency use authorization suggest an increased risk of GuillainBarré syndrome during the 42 days following
J J Covid Shot To Carry Guillain Barre Warning Chemanager
Guillain barre syndrome and covid vaccine fda
Guillain barre syndrome and covid vaccine fda- National and international vaccine guidelines did not preclude patients who have previously been diagnosed with GuillianBarré Syndrome (GBS) from receiving the COVID19 vaccine 1,2 However, previous association between vaccines and GBS raises the level of caution and hesitancy among clinicians and patients regarding administering the vaccine People receiving the Johnson and Johnson COVID19 vaccine could be at increased risk for developing GuillainBarré syndrome, the US Food and Drug Administration (FDA) is expected to announce as




Fda Warns J J Covid 19 Vaccine Raises Risk Of Guillain Barre Syndrome Complication Wsj
A Food and Drug Administration (FDA) fact sheet distributed with the Johnson & Johnson (J&J) vaccine will now contain warning language about a small increased risk that vaccine recipients have of developing GuillainBarré syndrome (GBS), a neurologic disorder that can cause progressive, but usually reversible, paralysis FDA continues to work with its partner in vaccine safety surveillance, the CDC, to monitor reports of GBS following vaccination with the Janssen COVID19 Vaccine GuillainBarré Syndrome (GBS) after Janssen COVID19 Vaccine Vaccine Adverse Event Reporting System (VAERS) Meeting of the Advisory Committee on Immunization Practices (ACIP) Office of Biostatistics and Epidemiology (OBE) FDA Center for Biologics Evaluation and Research (CBER)
On July 13, the US Food and Drug Administration (FDA) announced revisions to its vaccine recipient and vaccination provider fact sheets for the J & J vaccine US health regulators have warned that Johnson & Johnson 's Covid19 vaccine may be related to an increased risk of the rare neurological disorder known as Guillain–Barré syndrome The risk of this FDA To Attach Warning for GuillainBarre Syndrome to Johnson & Johnson COVID19 Vaccine The risk for developing the rare neurological condition are low following vaccination, with the FDA noting the benefits of vaccination far outweigh the potential risks Officials with the FDA are planning to attach a warning for GuillainBarre syndrome to the Johnson & Johnson
On 13 and July 21, the COVID19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) met virtually to discuss rare reports of GuillainBarré Syndrome (GBS) following vaccination with the Janssen and AstraZeneca COVID19 vaccines Both vaccines use an adenovirus platform as their backboneGBS is a rare immune system GuillainBarré syndrome, or GBS, was recently described as a rare side effect following receipt of adenovirusbased COVID19 vaccines, such as the J&J/Janssen version used in the US GBS has also been found to be a rare consequence of influenza vaccination About 100 preliminary reports of GuillainBarre syndrome have been detected after 128 million doses of the J&J vaccine were administered, the Centers for Disease Control and Prevention said in a



Covid Risque Accru De Developper Le Syndrome De Guillain Barre Avec Le Vaccin Johnson Johnson Les Echos




Syndrome De Guillain Barre Symptomes Vaccin Covid Pronostic
Guillain barre syndrome covid johnson and johnson Fda Adds Warning About A Nerve Condition To The Johnson Johnson Covid 19 Vaccine The Verge Us Warns Of Link Between J J Vaccine And Guillain Barre Syndrome World English Edition Agencia Efe F D A Attaches Warning Of Rare Nerve Syndrome To Johnson Johnson Covid Vaccine The New York Times Covid could be an occasional trigger of GuillainBarré syndrome (GBS), a study has claimed The autoimmune disease, which can leave patients paralysed and in crippling pain, has also been linked CDC 100 People Vaccinated With J&J COVID Shot Developed GuillainBarre The CDC says while most people fully recover from GBS, some experience permanent nerve damage or even die from disorder




Fda Johnson Johnson Covid 19 Vaccine Tied To Guillain Barre Syndrome



R I Approves Moderna Covid 19 Vaccine For Teens Ahead Of Fda Authorization The Boston Globe
Explained GuillainBarré and vaccines What you need to know Johnson & Johnson's beleaguered Covid19 vaccine may be associated with a small increased risk of GuillainBarré syndrome, a FDA warns on potential Johnson & Johnson COVID19 vaccine link to rare disorder The warning comes after 100 preliminary reports of GuillainBarre occurred against a backdrop of about 125 million FDA Warns J&J Covid19 Vaccine Raises Risk of GuillainBarré Syndrome Complication The rare neurological condition is a disorder in which the




F D A Attaches Warning Of Rare Nerve Syndrome To Johnson Johnson Covid Vaccine The New York Times




S France24 Com Media Display 79e5b7a6 7c 11eb A1ab a964fe W 1280 P 16x9 J 26j Webp
The Food and Drug Administration warned on Monday that Johnson & Johnson's coronavirus vaccine can lead to an increased risk of a rare neurological condition known as GuillainBarré syndrome The FDA has said that Johnson & Johnson's COVID19 vaccine may lead to an increased risk for GuillainBarré syndrome The New York Times reported on The condition, known as GuillainBarré syndrome, is a form of progressive paralysis and is generally reversible FDA vaccine regulators argue against Covid19 vaccine




J J Covid Vaccine May Pose Small Possible Risk Of Rare Neurological Syndrome Cdc Says Cbc News




Johnson Johnson In Discussion With Fda Regarding Covid 19 Vaccine Side Effects Reuters
In December , two vaccines have been approved in the United States for the prevention of COVID19 We report a case of GuillainBarre Syndrome (GBS) after receiving the first dose of Pfizer COVID19 vaccine Keywords neurologic complications, guillainbarre syndrome (gbs), covid19 vaccine IntroductionIn July 21, the Food and Drug Administration (FDA) announced that a small number of people who received the Johnson & Johnson COVID19 vaccine were later suspected of developing GBS Out of a total of 128 million doses of this vaccine administered in the US, there have been around 100 reports of suspected GBS1 day ago In November 13, I received a flu shot, and 10 days later I came down with GuillainBarre syndrome I was paralyzed for six months Half of my doctors say I should take the COVID vaccine




Johnson Johnson S Covid 19 Vaccine Has Another Problematic Side Effect




Coronavirus Johnson Johnson S Covid Shot Gets Fda Warning About Rare Immune Disorder
The US Food and Drug Administration on Monday warned of an increased risk of GuillainBarre, a rare neurological disorder, following the Johnson & Johnson vaccine after 100 preliminary cases of The FDA added a warning that Johnson & Johnson's COVID19 vaccine may trigger GuillainBarré syndrome (GBS) in a small number of people in a letter sent to the manufacturer on Monday Of the 12 The CDC says the agency has received about 100 reports of GuillainBarre syndrome among 128 million doses of the Johnson & JohnsonJanssen COVID19 vaccine




Guillain Barre Syndrome And The Johnson Johnson Vaccine What To Know The New York Times




Syndrome De Guillain Barre Causes Symptomes Vaccin Covid Traitement
The US Food and Drug Administration (FDA) has released a new warning that the onedose Johnson & Johnson (Janssen) COVID19 vaccine couldWe study 6,187 people who take Tila or have Guillain barre syndrome No report of Guillain barre syndrome is found in people who take Tila The phase IV clinical study is created by eHealthMe based on reports from the FDA, and is updated regularly The Food and Drug Administration on Monday added a warning to the Johnson & Johnson coronavirus vaccine, saying the shot can lead to an increased risk of a rare neurological condition Driving the news Although the chance of developing Guillain–Barré syndrome is "very low," the neurological disorder has occurred in some people who have received the J&J vaccine, the FDA



Vaccin Johnson Risque Accru De Developper Le Syndrome De Guillain Barre Liberation




Covid Vaccine Updates Fda Warns Of Rare Guillain Barre Syndrome Reaction To Johnson Johnson Coronavirus Vaccine Abc7 New York
The Johnson & Johnson coronavirus vaccine now comes with a warning on its label about an increased risk of nerve damage known as GuillainBarré syndrome (GBS) It has primarily been seen in menThe other half say I shouldn't FDA has added a warning to Johnson & Johnson's Covid19 vaccine fact sheet after receiving preliminary reports of patients developing the rare neurological condition GuillainBarré syndrome after




Fda Will Attach Warning Of Rare Nerve Syndrome To Johnson Johnson S Covid 19 Vaccine Baltimore Sun



Syndrome De Guillain Barre Le Trouble Neurologique Qui Touche Certaines Personnes Vaccinees Contre Le Coronavirus c News Afrique
The US Food and Drug Administration added a new warning to documentation for Johnson & Johnson's Janssen COVID19 vaccine Monday after 100 recipients reported GuillainBarré syndrome 545 pm ET, FDA updates label of J&J Covid19 vaccine to warn of potential GuillainBarre syndrome From CNN's Amanda Sealy and Maggie FoxWe report the first case of Guillain–Barre Syndrome after receiving the second dose of the Pfizer COVID19 vaccine, in a 42yearold woman presenting with




Covid 19 Le Vaccin Janssen Associe A Un Surrisque De Guillain Barre Selon La Fda Le Generaliste



Fda Warns Of Guillain Barre Risk For Johnson Johnson Vaccine
On July 12, the FDA requested that Janssen Pharmaceutical Companies of Johnson & Johnson (J & J) add a warning to its COVID19 vaccine Emergency Use Authorization Fact Sheet for Healthcare Providers regarding the risk of a potential side effect known as Guillain Barré Syndrome "Guillain Barré syndrome (a neurological disorder in which the Officials at the US Food and Drug Administration warned of a rare but serious neurological disorder linked to Johnson & Johnson's coronavirus vaccine Physicians Offer Expanded Warning about GuillainBarré after COVID Jab TUCSON, Ariz, /PRNewswire/ The Food and Drug Administration (FDA) has issued a warning about a threeto



Fda Warning About J J Vaccine Creates More Challenges For Fighting Covid Crisis



Johnson Johnson Covid 19 Vaccine May Lead To Guillain Barre Syndrome Fda To Issue Warning
Dear Dr Roach I have multiple sclerosis, Addison's disease, rheumatoid arthritis and lupus I am on steroids daily as well as potent immunosuppressives In November 13, I received a flu shot, and 10 days later I came down with GuillainBarre syndrome I was paralyzed for six months Half of my doctors say I should take the COVID vaccine; The United States Food and Drug Administration (FDA) has warned that the COVID19 vaccine developed by Johnson & Johnson may be linked to a small increased risk of GuillainBarré syndrome, a But, since the COVID19 vaccines don't trigger those symptoms, they should be safe to receive "The vast majority of vaccines are totally safe




Fda Adds Warning About Rare Reaction To J J Covid 19 Vaccine Wgn Tv




Fda Warns Of An Increased Risk Of Guillain Barre Syndrome Following Vaccination With The J J Vaccine Healthcare Finance News
The new findings of GuillainBarre Syndrome (GBS) developing approximately two weeks after receipt of the vaccine has predominantly been seen in men over age 50On , the FDA announced revisions to the vaccine recipient and vaccination provider fact sheets for the Johnson & Johnson (Janssen) COVID19 Vaccine to include information pertaining to an observed increased risk of GuillainBarré syndrome following vaccination 1 GuillainBarré syndrome is a rare disorder where the body's immune system damages nerveFDA did not identify an increased risk for GBS in its evaluation of the clinical trials data regarding Shingrix, which FDA evaluated prior to the vaccine's approval in 17



Fda Adds Warning To J J Covid 19 Vaccine After Gbs Cases Cgtn




Foundation Hosted Patient Listening Session On Guillain Barre Syndrome With The Fda Gbs Cidp Foundation International
The Johnson & Johnson (J&J) coronavirus vaccine may be linked to rare but serious cases of GuillainBarré syndrome, an autoimmune disorder in which immune cells attack the nervous system, the US Food and Drug Administration has warned1 The FDA's warning on 12 July came three days after the European Medicines Agency's safety committee recommended adding a GuillainGuillain barre syndrome and covid vaccine canada Guillain barre syndrome and covid vaccine canada The vaccine has been approved in Canada but never used The US Centers for Disease Control and Prevention said on Monday that it has received rare reports of GuillainBarré syndrome among Update The FDA added warnings about GuillainBarre




Stakeholder Call Guillain Barre Syndrome Gbs Covid 19 Vaccine Updates 7 15 21 Youtube




Www Usinenouvelle Com Mediatheque 4 0 7 6x598 C Jpg




J J Covid Shot To Carry Guillain Barre Warning Chemanager




Fda Links J J Vaccine To Rare Cases Of Guillain Barre Drug Discovery And Development




Fda Warns About Post Covid Vax Guillain Barre Syndrome Medpage Today




Www Sudinfo Be Sites Default Files Dpistyles V2 Ena Sp 16 9 Illustration Principale 21 07 12 Node Public 21 07 12 Bz 1 000 G6lihe4hh 1 0 Jpg Itok Nitdm9ta




J J Covid Vaccine Warning Fda Adds Info On Rare Reaction Risk 9news Com




Pharmacy Times Fda To Attach Warning For Guillain Barre Syndrome To Johnson Johnson Covid 19 Vaccine Learn More T Co 8dxxouuzyf T Co Pkodk1dhbd



Johnson Johnson Covid Vaccine And Guillain Barre Everything To Know



Fda Adds Guillain Barre Syndrome Warning To J J Covid Vaccine Axios
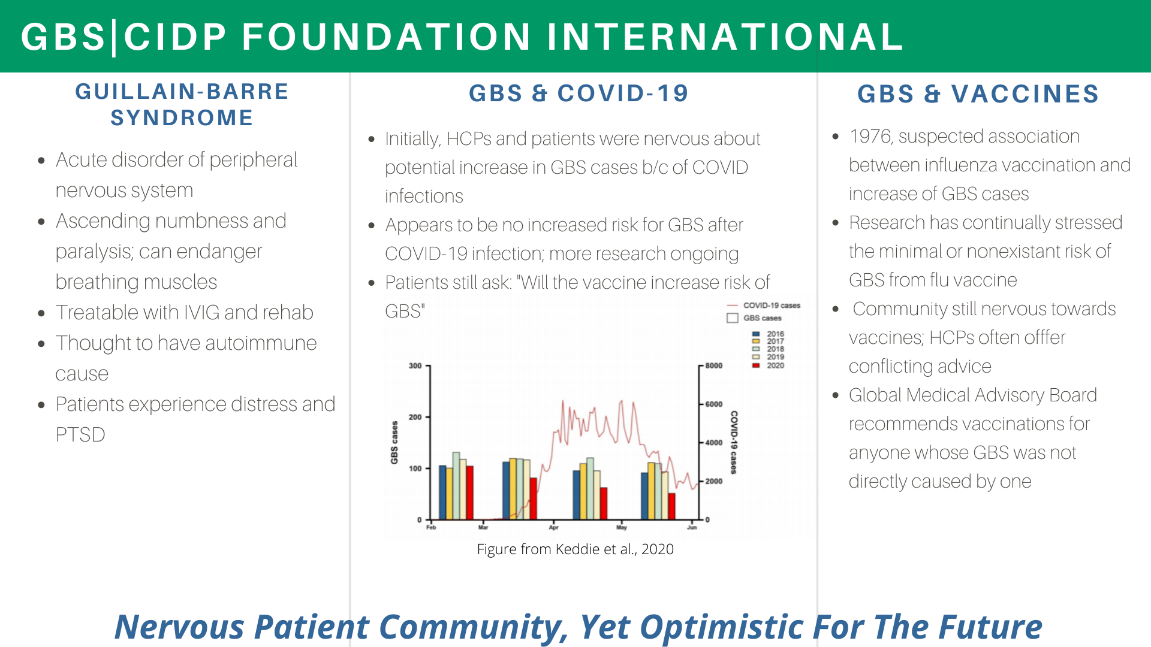



Gbs Cidp Foundation Staff Patients Collaborate With Fda Gbs Cidp Foundation International




Explained What Is Guillain Barre Syndrome Why It S Being Linked To J J Vaccine



In Response To Rare Guillain Barre Cases Fda Plans New Warning For J J Covid 19 Vaccine Biospace




Fda Adds New Warning Of Rare Nerve Syndrome To Johnson Johnson Covid 19 Vaccine



New Covid 19 Vaccine Warnings Don T Mean It S Unsafe They Mean The System To Report Side Effects Is Working



Fda Updates J J Covid 19 Vaccine Label To Include Rare Disorder



J J Vaccine Side Effects Guillain Barre Syndrome And More Nbc Chicago




U S Puts New Warning On J J Coronavirus Vaccine For Autoimmune Disorder Reuters




Fda Warns Guillain Barre Syndrome May Be Linked To Johnson Johnson Vaccine




Fda Adds Warning Of Rare Neurological Reaction To J J Covid 19 Vaccine Business Standard News



Fda Will Announce Rare Incidence Of Guillain Barre Linked To Johnson Johnson Vaccine



Fda Adds Warning About Rare Reaction To J J Covid 19 Vaccine




Fda Issues Guillain Barre Warning On J J Covid 19 Vaccine



What Are The Symptoms Of Guillain Barre Syndrome Should I Still Get The J J Vaccine Marketwatch




Lire Comparaison Des Effets Secondaires Du Vaccin Covid 19 Avec La Premiere Liste A Surveiller De La Fda Fr24 News France




European Commission We Are Starting To Turn The Page On A Difficult Chapter We Have Authorised The First Covid 19 Vaccine For Use In The Eu More Vaccines Will Follow
:no_upscale()/cdn.vox-cdn.com/uploads/chorus_image/image/69574976/1232865115.0.jpg)



Fda Adds Warning About A Nerve Condition To The Johnson Johnson Covid 19 Vaccine The Verge




The Link Between The Johnson Johnson Vaccine And Guillain Barre Syndrome Shape



Fda Warns About J J Covid Vaccine And Guillain Barre Syndrome Risk Health Com




Www Leparisien Fr Resizer Mygsjc90yxmswyfe8uwly9x1dmc Arc Anglerfish Eu Central 1 Prod Leparisien Public Jvuaaimixrgjbpydhu62dvffoq Jpg




Learn More About Covid 19 Vaccines From The Fda Fda



Vaccine Guillain Barre Syndrome A New Rare Side Effect Associated With Astrazeneca Sortiraparis Com




J J Covid 19 Vaccine May Trigger Neurological Condition In Rare Cases




Had The J J Vaccine What You Should Know About Latest Risk



Www Openaccessgovernment Org Wp Content Uploads 21 07 Dreamstime Xl Scaled Jpg




Case Reports Describe Unusual Guillain Barre Variants Following Covid 19 Vaccination




What Do You Need To Know About The Guillain Barre Disease And Johnson And Johnson Vaccines Health News Firstpost



2




Cdc Fda Looking Into Risk Of Rare Nerve Complication After Johnson Johnson Covid 19 Vaccine




Janssen Vaccine Increases Risk Of Guillain Barre Syndrome




Images Midilibre Fr Api V1 Images View 60edb4b68fe56f7e927c32c6 Hd Image Jpg V 1



Qu Est Ce Que Le Syndrome De Guillain Barre Associe Au Vaccin Johnson Johnson



Rare Cases Of Guillain Barre Syndrome Reported In Janssen Covid 19 Vaccine




Fda Adds J J Covid 19 Vaccine Warning Cidrap




Fda Warns J J Covid 19 Vaccine Raises Risk Of Guillain Barre Syndrome Complication Wsj




New Covid 19 Vaccine Warnings Don T Mean It S Unsafe They Mean The System To Report Side Effects Is Working



J J Covid Vaccine Warning Fda Adds Info On Rare Reaction Risk Weareiowa Com



Johnson Johnson Vaccine And Guillain Barre Fda Adds New Warning Related To Rare Autoimmune Disorder The Washington Post




The Fda Warns That Johnson And Johnson Covid Vaccine May Be Linked To Guillain Barre Syndrome Wusa9 Com



J J Warning And The Guillain Barre Syndrome 11alive Com




Neuropsychiatrist On Incredibly Rare Condition Linked To Johnson Johnson Covid 19 Vaccine Cbs News




Johnson Johnson Vaccine And Guillain Barre Fda Adds New Warning Related To Rare Autoimmune Disorder The Washington Post




Fda Adds Warning About Rare Reaction To J J Covid 19 Vaccine




Fda Expected To Issue New Warning On J J Covid Jab Business And Economy News Al Jazeera




Fda Warns Of Possible Rare Association Between Johnson Johnson Vaccine And Guillain Barre Syndrome




Guillain Barre Syndrome Read Guillain Barre Syndrome News Photos Videos




J J Covid Vaccine Warning Fda Adds Info On Rare Reaction Risk Fox61 Com




Covid 19 Vaccine Faq Metropolitan Pediatrics Metropolitan Pediatrics



Cureus Neurological Complications Of Covid 19 Guillain Barre Syndrome Following Pfizer Covid 19 Vaccine



The Johnson Johnson Vaccine And Guillain Barre Syndrome What Michigan Needs To Know Bridge Michigan




Fda Adds Guillain Barre Syndrome Warning To J J Covid Shot Politico




What Is Guillain Barre Syndrome Cbs19 Tv




Johnson Johnson Covid 19 Vaccine Holds The Risk Of Paralysis Fda Articles




What To Know About Guillain Barre And The Covid Vaccine Cleveland Clinic



Chart Covid Vaccines Compared



Guillain Barre Syndrome Johnson And Johnson Vaccine Fit Increase Risk Of Nerve Disorder Us Fda Warn c News Pidgin
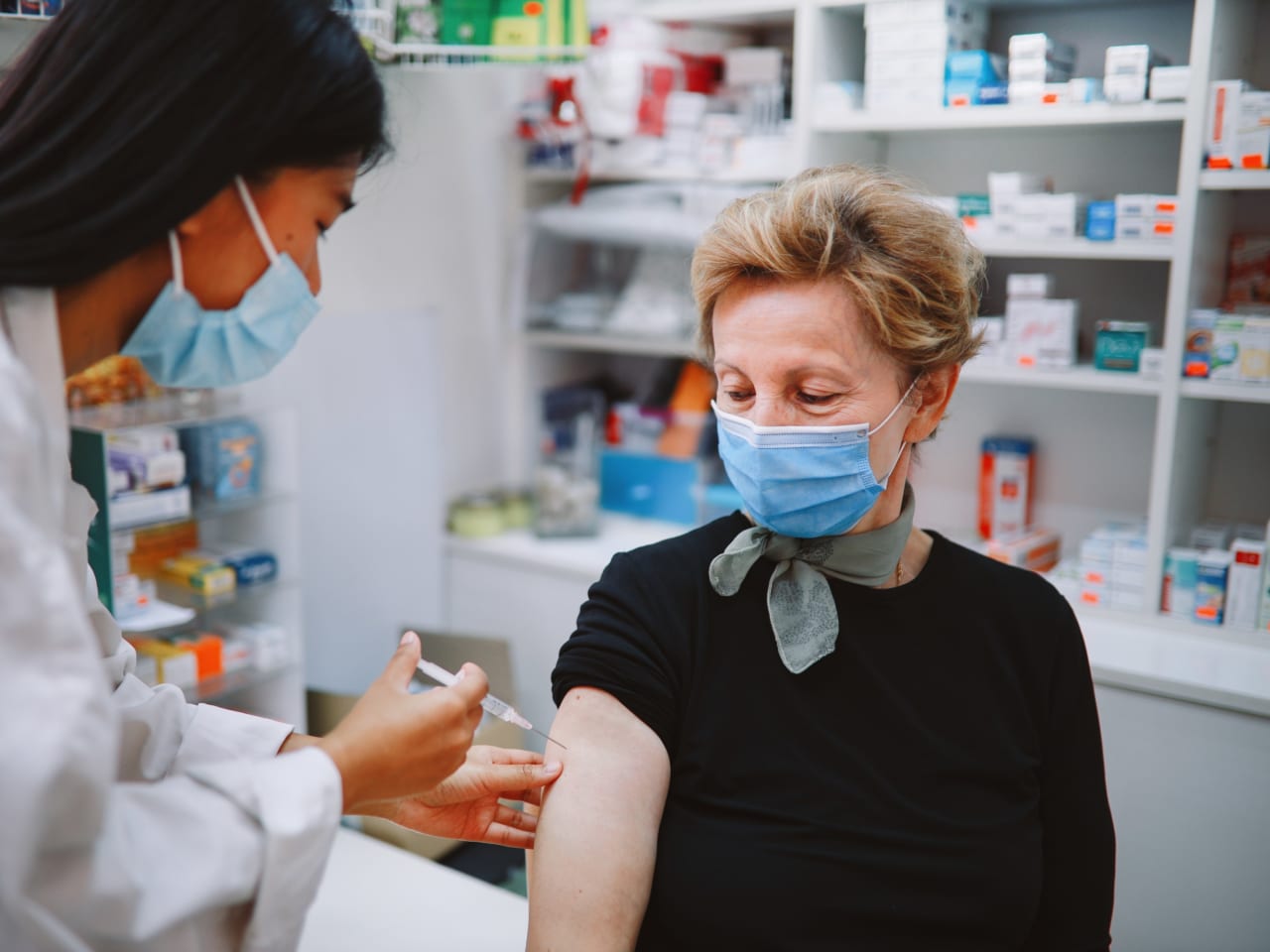



J J Vaccine And Guillain Barre Syndrome Information On The Fda Warning News Yale Medicine




J J Covid Vaccine Fda To Announce New Warning Related To A Rare Autoimmune Disorder Report Says




Static Dw Com Image 401 Jpg



Fda Adds Warning Of Rare Reaction Risk To J J Covid 19 Vaccine The Hindu



Vaccin De Johnson Johnson Contre Le Covid 19 Mise En Garde Sur Un Risque Accru D Une Rare Maladie Neurologique Aux Etats Unis




Photos Lci Fr Images 1280 7 Janssen Ok 2cb28a 0 1x Jpeg



Vaccin Johnson Johnson Risque Accru De Developper Le Syndrome De Guillain Barre Selon La Fda Sortiraparis Com




What Is Guillain Barre Syndrome The Rare Condition That The Fda Have Linked To The Johnson Johnson Vaccine As Com




Vera Jourova Recovery From Coronavirus Can Be Accomplished Only By Upholding To Fundamentalrights European Values Are The Only Way Out Of This Crisis I Nominate Zuzanacaputova To Join This Challenge



Fda Adds Guillain Barre Warning To J J Vaccine What To Know Time



0 件のコメント:
コメントを投稿